Hypertension Treatment Simulation Software Download Pc Software
- Written report protocol
- Open Access
- Published:
Conquering hypertension in Vietnam—solutions at grassroots level: study protocol of a cluster randomized controlled trial
Trials volume 21, Article number:985 (2020) Cite this commodity
Abstract
Background
Vietnam has been experiencing an epidemiologic transition to that of a lower-centre income country with an increasing prevalence of non-infectious disease. The key risk factors for cardiovascular disease (CVD) are either on the rising or at alarming levels in Vietnam, particularly hypertension (HTN). Inasmuch, the burden of CVD will keep to increment in the Vietnamese population unless effective prevention and control measures are put in place. The objectives of the proposed projection are to evaluate the implementation and effectiveness of 2 multi-faceted customs and clinic-based strategies on the control of elevated claret pressure (BP) among adults in Vietnam via a cluster randomized trial design.
Methods
Sixteen communities will be randomized to either an intervention (viii communities) or a comparison group (8 communities). Eligible and consenting adult written report participants with HTN (n = 680) will be assigned to intervention/comparison condition based on the community in which they reside. Both comparison and intervention groups will receive a multi-level intervention modeled after the Vietnam National Hypertension Program including education and practice change modules for health care providers, attainable reading materials for patients, and a multi-media customs sensation program.
In addition, the intervention group merely will receive three carefully selected enhancements integrated into routine clinical care: (1) expanded customs health worker services, (2) abode BP self-monitoring, and (3) a "storytelling intervention," which consists of interactive, literacy-advisable, and culturally sensitive multi-media storytelling modules for motivating beliefs alter through the power of patients speaking in their own voices. The storytelling intervention volition exist delivered by DVDs with serial installments at baseline and at 3, 6, and 9 months after trial enrollment. Changes in BP volition exist assessed in both groups at several follow-up time points. Implementation outcomes volition be assessed as well.
Discussion
Results from this full-scale trial will provide health policymakers with practical evidence on how to combat a key risk cistron for CVD using a viable, sustainable, and cost-effective intervention that could be used as a national programme for controlling HTN in Vietnam.
Trial registration
ClinicalTrials.gov NCT03590691. Registered on July 17, 2018. Protocol version: half dozen. Appointment: August 15, 2019.
Groundwork
Epidemiologic transition in Vietnam, cardiovascular disease, and hypertension
Vietnam is undergoing an epidemiological transition with the morbidity and bloodshed from non-infectious disease having risen quickly over the final several decades [1, 2]. This transition tin can be attributed to changes in population size, socio-demographic characteristics, and increases in life expectancy [1,2,3,4]. Cardiovascular disease (CVD) is at present the leading cause of death in Vietnam, accounting for 30% of all deaths annually in 2010 [five]. Major chance factors for CVD including hypertension, diabetes, unhealthy dietary practices, and overweight/obesity are either on the rising or at alarming levels in Vietnam [2, 6, 7]. National data showed that the prevalence of hypertension (HTN) was more than 40% for those 50–69 years old and the general population consumed high levels of sodium in their diet in 2016 [vii]. Although antihypertensive medications are off-patent, widely available across the country, and are covered by public health insurance (more than 80% of Vietnamese accept health insurance), the sensation and management of hypertension are far from optimal [8]; this is due to many factors including lack of regular screening and patient self-management strategies (e.chiliad., medication adherence, lifestyle modifications). Inasmuch, the brunt of CVD will continue to increase in the Vietnamese population unless effective prevention and command measures are put in place.
The wellness care arrangement in Vietnam is organized into four levels, which include the central, provincial, district, and commune (customs) level. The lowest level includes the community wellness centers (CHCs), which are responsible for providing primary health care and outpatient services, including implementation of national health programs. There is typically one CHC per community, and at each CHC, there are approximately x–15 community health workers (CHWs). These individuals are "natural helpers" without medical degrees who serve equally health advocates for their community [9], educators, and problem-solvers, and they provide valuable linkages to available community resources [10]. Contempo studies have documented the positive role of CHWs in improving HTN command through better home monitoring, appointment keeping, medication adherence, and health care use [eleven,12,13]. CHWs are present in our partnering rural clinics and were an integral part of our previous work [14,15,16].
Between 2014 and 2016, a feasibility cluster trial of a storytelling intervention was conducted in Hung Yen province, Vietnam, by members of the study team [14,15,xvi]. The report included 160 patients with HTN with a mean age of 66 years and 54% were men. Between baseline enrollment and the iii-month follow-upwards, systolic claret pressure (BP) declined by 8.2 mmHg (95% CI iv.1–12.2) in the storytelling grouping and by 5.v mmHg (95% CI 1.4–9.5) in the comparison group; HTN medication adherence increased in the storytelling group and declined in the comparing group. Building on the findings of this feasibility trial, we proposed a total-scale cluster randomized controlled clinical trial to evaluate the implementation and effectiveness of two multi-faceted customs and dispensary-based strategies on the control of elevated blood force per unit area among adult men and women in Vietnam.
Methods
Intervention approaches and implementation framework
Patient-centered interventions
Interventions focused on how patients may reduce their run a risk of CVD through lifestyle changes including weight control, increased concrete activity, tobacco avoidance, moderate intake of table salt and alcohol, and adherence to prescribed medications [17,18,19].
Habitation claret pressure self-monitoring
Home self-blood pressure monitoring enhances patient self-management and empowerment. Compared with role-based readings, home blood pressure level monitors may provide better prognostic information virtually CVD outcomes [20]. Several studies have documented the value of home blood pressure monitoring in improving HTN command [21,22,23] and increased patient adherence to blood pressure lowering medications [24, 25].
Community health workers
Community health workers (CHWs) are "natural helpers" who serve as health advocates for their local communities [9]. A CHW may serve as an educator and problem-solver and provide a valuable linkage to community resources and health promotion activities [x, 26]. Recent studies have documented the positive office of CHWs in improving HTN control [11, 12, 27], and CHWs are nowadays in our partnering rural clinics and were an integral office of our previous piece of work [14,fifteen,xvi].
Intervention model
Wagner's original Chronic Intendance Model [28], subsequently expanded [29], provides a conceptual foundation for addressing multi-level barriers to HTN command (Fig. one). Although we do non have the capacity in rural Vietnam to implement all components of this model, information technology notwithstanding provides a systematic framework for organizing our multi-level intervention. This model has been successfully used for HTN direction [12, xxx, 31], and nosotros have adapted it for the rural Vietnamese setting to guide our intervention strategy. Our intervention components map directly to the Chronic Intendance Model in the domains of patient self-management back up (storytelling, abode blood force per unit area monitoring), clinician determination support (medication management as part of the Vietnam National HTN Program), delivery organisation blueprint (standardized blood pressure measurement), clinical information systems (tracking software available through the Vietnam National HTN Program), health care organisation (leadership buy-in), and community resources (CHWs).
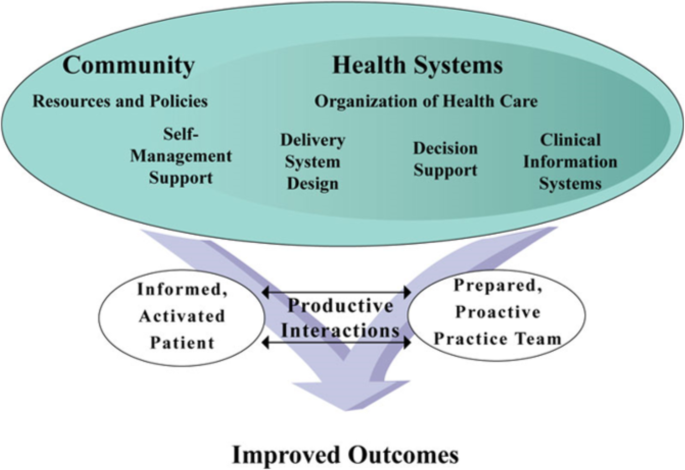
The adapted Chronic Care Model
Implementation framework
While we considered several implementation frameworks [32,33,34], we chose the Promoting Activity on Research Implementation in Health Services (PARiHS) model because nosotros will be implementing a multi-level model in a de-centralized system with semi-democratic clinical delivery systems. PARiHS relies on three interactive elements: show, context, and facilitation [35,36,37], which play key roles in our intervention.
Our specific aims are equally follows:
- 1.
Acquit a pre-implementation local needs cess and formative planning in 16 partnering communes in Hung Yen province, Vietnam, leading to a context-specific protocol for implementing the Vietnam National Hypertension Program in both intervention and comparison communities and proposed enhancements (expanded CHW services, dwelling blood force per unit area self-monitoring, and storytelling in the eight intervention communities just).
- two.
Implement a cluster randomized controlled trial (RCT) (Blazon I Hybrid Implementation Design) of 16 communities and 680 patients with HTN randomized to either an intervention group (Vietnam National Hypertension Program plus iii trial enhancements) or a comparison group (Vietnam National Hypertension Programme lonely).
- 3.
Compare the effectiveness and implementation success of the two approaches using data from multiple sources at multiple points in fourth dimension, including blood pressure level measurements, patient surveys, and interviews with clinic personnel and clinicians. Our main study hypothesis is as follows: at 12 months post-randomization, participants in the intervention group volition have a greater mean reduction in their levels of blood pressure than those in the comparison grouping.
Study setting
The study volition be conducted in the Blood-red River Delta Region in northern Vietnam. In this region, communes (communities) in Hung Yen province were selected based on their general representativeness. Hung Yen province has a population of approximately i.3 million, organized into 10 districts and 161 communes. In Vietnam, the health system is organized into four levels, namely central, provincial, district hospital, and the lowest level, which includes the community health centers (CHCs) that are responsible for providing chief health care and outpatient services. Patients with HTN are typically treated and managed at the district health centers unless they need to be referred for a higher level of care.
Needs assessment study
During the first 6 months of funding, we will conduct a qualitative written report—needs assessment survey at participating study sites. We recognize the multi-level ecological context of our intervention with layers of influence on health status, health behaviors, and behavioral changes beyond the individual. These include (1) customs (individuals and families), (two) participating organizations, (three) socio-cultural environment, (4) concrete congenital environment, and (5) the broader policy surroundings.
The needs assessment survey will exist based on the triangulation of multiple data sources, including databases documenting the prevalence and control of HTN in the study communes, and semi-structured interviews with clinicians, dispensary staff, CHWs, and customs members. The structured interviews will ascertain perceptions of clinicians and clinic leadership about the evidence for treating HTN (testify), strengths and limitations of the electric current environment for implementing new tools for HTN control proposed as part of our intervention (context), and specific approaches needed to overcome barriers to blood pressure control (facilitation).
As part of the needs assessment survey, we will perform 21 full semi-structured, individual interviews in a randomly selected subset of iii communities from the intervention sites and iii communities from the comparison sites. This will consist of semi-structured interviews with clinicians, nurses, and leadership at the health centers and interviews with patients with uncontrolled HTN. We volition work closely with the Department of Wellness in Hung Yen province to identify stakeholders who sympathise and are involved in HTN direction in the community and physicians who take managed HTN patients at the provincial and district hospitals to participate in the written report. Patients with uncontrolled HTN volition be referred by their physicians at the local hospitals. Cursory structured qualitative assessments will be conducted at all remaining sites via focus group discussions (FGDs). We will conduct 9 FGDs at 3 study communities.
These interviews will be repeated on three occasions in study years 1, three, and 5. The 2d and third rounds of interviews will employ a similar design to the kickoff round, and at that place several boosted questions will assess the progress of the study implementation. We anticipate that subsequently intervention implementation, the gaps in HTN management found in the needs assessment study will be narrowed to a greater degree in the intervention grouping. Information on intervention acceptability, appropriateness, feasibility, and allegiance volition be collected likewise.
A cluster randomized controlled trial (Type I Hybrid Implementation Design)
A total-scale cluster RCT will be conducted in Hung Yen province, Vietnam. Sixteen communities (communes) and 680 patients with HTN volition be randomized to either an intervention (Vietnam National Hypertension Programme plus 3 trial enhancements) or a comparison group (Vietnam National Hypertension Program solitary) (Fig. 2). The schedule of enrolment, interventions, and assessments is presented in Fig. 3.
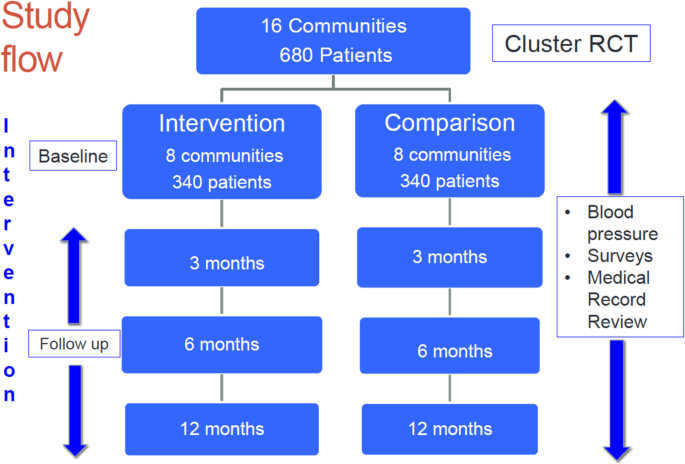
Report catamenia
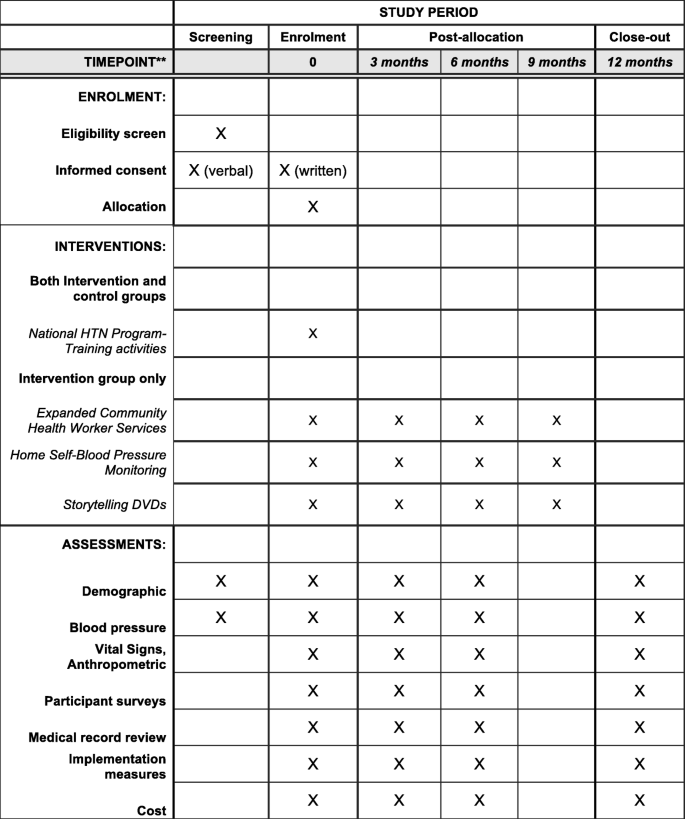
The schedule of enrolment, interventions, and assessments
Study sites
A total of 16 eligible communes in 4 districts in Hung Yen province will be randomly assigned to either the intervention (n = 8) or the comparing group (due north = 8). Each of the selected communes satisfies the post-obit criteria: (1) have a CHC with a medical doctor, (ii) are not currently participating in other studies for HTN control, and (3) have a minimum geographic separation of 12 km (7 miles) from all other report communities to minimize possible contamination. Eligible and consenting trial participants with uncontrolled HTN (n = 680; approximately 43 patients per commune) will be assigned to intervention versus comparing status based on the customs in which they reside.
Participant eligibility
To be enrolled in our pilot study, consenting adult men and women must fulfill each of the following criteria: (1) be a resident of the selected commune and have no plans for moving during the next 12 months subsequently trial enrollment; (2) be anile 25 years or older, a cutoff which is normally used in population-based surveillance studies of HTN in Vietnam [6, 8]; (three) have a diagnosis of uncontrolled HTN co-ordinate to the eighth Articulation National Commission of Loftier Claret Pressure (JNC viii) [38]; (4) not be cognitively dumb (as assessed by study physicians); (five) not be a "story teller" used to develop the intervention; (6) not be a family member of another participant in the report; and (7) not meaning.
In add-on, those diagnosed with elevated BP for the offset time volition be invited for re-measurement of their BP over the next 2 weeks (minimum of 1 week apart) afterward their initial CHC visit. If their average BP remains elevated, these persons will be invited to participate in the trial. Trained study nurses at written report sites will obtain informed consent from eligible patients. Both newly diagnosed and prevalent patients with HTN (treated or otherwise) will be enrolled. If they are not treated at the time of trial enrollment, they will exist referred to their commune health centers for a follow-upwardly test and initiation of treatment, which will be covered by public wellness insurance. Members of the study team will piece of work closely with doctors at the district health centers to follow upwards these patients.
Study recruitment and randomization
Sixteen communities will be randomly assigned to either the intervention or the comparison status stratified by districts using the STATA program. In each district, two communes will be randomly assigned to the intervention group and 2 communes will be randomly assigned to the comparison grouping. Patients volition be recruited from the customs setting. Screening events to identify patients satisfying our written report criteria volition exist conducted at local CHCs. A second screening visit volition be scheduled for eligible patients with elevated blood pressure (BP) values (systolic BP ≥ 140 mmHg, or diastolic BP ≥ ninety mmHg) 2 weeks later, at which fourth dimension their BP will exist re-measured and written informed consent volition be obtained. Individuals who are found to have elevated BP at the time of clinic screening and are not willing to participate in the study will be referred for usual care at local district health centers.
The recruitment will rely on CHWs embedded in both the dispensary and the customs, which volition provide credibility and trust. The CHWs and study nurses will offer BP screenings at their CHCs. With this approach, our recruitment goals will achieve in the allotted time.
Later enrollment, a reminder letter will be sent to all participants before each of the follow-up visits. One week earlier the scheduled follow-up visit, local staff will contact study participants by phone. Because the communes are small, it is easy to visit participant'southward homes. If patients miss their follow-up visit, local staff volition contact them by telephone to remind them to come into the CHC or visit their homes to measure their BP, if necessary. Patients can withdraw from the study at any time during the report period. If patients move out from their communes permanently, they volition exist considered equally dropping out from the study.
Intervention status and delivery
Detailed interventions and delivery methods are described in Table 1.
Vietnam National HTN Plan (intervention and comparing groups)
The Vietnam National HTN Programme is role of a comprehensive Vietnam National Strategy on Prevention and Control of Not-Communicable diseases [39]. This multi-arm national strategy was approved by the Prime Government minister in 2015, merging several national programs into a cohesive, integrated approach. The Vietnam National HTN Program was authorized in 2008 including diverse training sessions about HTN prevention and management for physicians and nurses, and a comprehensive fix of patient educational activity materials written in a culturally and literacy-appropriate manner. Multi-media community service announcements accept been prepared for local television and radio stations and newspapers, and will be implemented in both study groups. There volition be a serial of training sessions for health intendance providers which will be carried out at local commune or provincial health departments in collaboration with the Vietnam Ministry building of Health.
Expanded community health worker services (intervention group only)
CHWs are currently embedded in the clinical system for each of our partnering CHCs and across the nation. A critical enhancement for the intervention grouping volition exist to support and strengthen their office in activating patients to more than actively manage their HTN through lifestyle changes and adherence to prescribed antihypertensive medication. CHWs volition be trained in motivational interviewing and structured trouble-solving. This behavioral alter counseling approach facilitates improvements in nutrition, exercise, adherence to medication regimens, tobacco use, and overall appointment in one's care [40]. CHWs without advanced health intendance training tin be safely trained in this dynamic approach to facilitate health behavior change with proven effectiveness [41, 42].
CHWs will also exist taught simple techniques to assist patients prepare goals for a number of lifestyle changes, including common salt and booze reduction, smoking abeyance, increased concrete activity, and enhanced medication adherence; they will also develop problem-solving strategies to achieve these goals. CHWs will exist taught how to work with patients to (1) engender appointment and delivery past self-identifying goals that are meaningful and consistent with their personal lives and family context; (2) promote feasibility past identifying a limited set of goals and small, accessible steps; (iii) provide educational resource; (4) institute a structure for accountability and support through regular review; and (5) link goal attainment to changes in self BP monitoring for reinforcement. These techniques were successfully used in our pilot work [15].
As in our pilot work, CHWs will be taught how to apply the storytelling intervention (below) to first conversations with their patients with elevated BP values. After each DVD of the storytelling intervention, CHWs volition see with the patient to review the material, arm-twist possible barriers to lifestyle changes and medication handling, and place strategies to overcome recognized barriers. CHWs will make bi-weekly patient dwelling house visits (i h) to resolve difficulties related to viewing the DVDs, and they will continue detailed logs of their patient interactions to help provide a qualitative sense of intervention effectiveness and propose approaches for improvement. At that place volition exist a series of training sessions for CHWs, which will be delivered locally in the collaboration with the Vietnam Ministry of Health.
Home self-blood pressure level monitoring (intervention group only)
Dwelling BP self-monitoring is the second enhancement for the intervention group. The intervention group volition receive free home BP measurement devices at the time of trial enrollment at their CHCs whereas patients in the comparison group will receive dwelling house BP monitors and a BP log after the study has ended. After obtaining informed consent, a trained CHW will instruct patients on how to utilize the BP measurement devices at dwelling house and how to record their BP readings in the BP log previously adult and implemented by the study team. Readings will initially exist taken in the forenoon subsequently arising and once more at dark before going to sleep. Patients will be advised about reading variability, cautioned well-nigh overreacting to a single elevated BP value, and given specific protocols for when to contact a health care provider should the need arise. Patients are routinely given a portable re-create of their medical record with instructions to bring it to future clinic appointments.
Storytelling intervention (intervention group only)
Our squad has previously designed, implemented, and airplane pilot tested such an intervention for improving HTN control in Vietnam [fourteen,xv,16]. The patient narratives included in the intervention materials include first-hand accounts from patients in their journey to gain command of their HTN, and the stories are complemented by additional formal information most HTN control. This Learn-More section volition be built on the stakeholders' opinions gathered via interviews and national experts in HTN control. For our newly proposed work, we will supplement this previously developed cloth with new patient stories to represent our expanded community base. We will develop four storytelling DVDs, the commencement to be delivered to intervention patients at the time of trial enrollment, with viewing in the clinic, followed past installments at iii, vi, and nine months to be viewed at home. All intervention participants will be provided with a DVD player and instructed in how to navigate the carte structure of the DVDs at enrolment. Later on each installment, nosotros will administer a post-media interview to ascertain the frequency and duration of viewing, change in behavioral intentions, and overall satisfaction with the intervention; these data will be used for the mediation analysis to depict implementation and mechanisms of intervention effectiveness. At 3 and 6 months later trial enrollment, a second and tertiary installment of the DVDs volition exist delivered at the patient's local CHC for home viewing by patients assigned to the storytelling intervention group. At 9 months after trial enrollment, the fourth DVD will be delivered at participant's dwelling house past the CHWs.
Nosotros will develop 2 short DVDs with Learn-More department only for the comparison grouping. Patients in the comparing grouping will receive a DVD role player and the first DVD at trial enrollment and the second DVD at month 6 at their local CHC.
For both groups, afterward viewing the DVDs, a follow-upwardly visit will be scheduled for a "postal service-media" interview and re-measurement of patient'southward BP by a trained CHW.
Sample size
Sample size calculations are based on our primary trial hypothesis with between-group differences in over-time changes in systolic BP equally the principal trial outcome. Our previous pilot work in rural Vietnam suggests that information technology is viable to achieve an over-time improvement of viii mmHg in systolic BP with a standard divergence of 18 mmHg. Analysis of pilot information revealed an intra-form correlation of 0.011 for the clustering of participants in communes for change in systolic BP. We showtime performed unadjusted sample size calculations that did not account for clustering of individuals within study site and did not inflate for possible losses to follow-up. For these calculations, nosotros set alpha mistake at 0.05 and examined a range of power from 0.8 to 0.nine based on the two-sided t exam with a common standard difference of 18, assuming that the mean improvement in systolic BP is 8 mmHg for patients in the intervention condition and 3 mmHg for those in the comparing status.
Side by side, we adapted these first-laissez passer sample size calculations to account for the clustering of participants. According to Donner: Due north adapted= N undjusted(1 + (m − one)r), in which N adjusted is the total sample size adjusted for clustering, Due north undjusted is the unadjusted full sample size, m is the unadjusted average cluster size (average number of patients/community), and r is the intra-form correlation (ICC) [43]. Finally, we inflated the resulting sample size by approximately ten% to business relationship for potential losses to farther follow-up. Information technology is important to notation that the planned analyses for this report will draw upon the power of longitudinal measurement, which will be more powerful than the higher up-presented estimates [14]. Data to inform these calculations were based on recently published piece of work and are summarized in Table 2.
In improver to the chief analyses described above, we also anticipate adequate power for the planned mediation analysis. For the mediation analysis, simulation studies revealed that a sample size of 500 is adequate to detect pathways with small standardized event sizes (as low as 0.14) at 80% power with methods described above [44].
Data collection
Data sources include the post-obit: (1) standardized BP and anthropometric measurements at baseline and at 3, 6, and 12 months afterwards trial enrollment; (2) quantitative participant surveys at baseline and at iii, 6, and 12 months later on enrollment; (3) post-media interviews after each installment of the storytelling intervention for the intervention grouping; (five) medical record review; (6) implementation data gathered by the inquiry coordinator and CHWs that will inform progress toward specific written report milestones; and (vii) semi-structured interviews for qualitative information.
Claret pressure level and anthropometric measurement
Every bit previously described, certified report nurses volition be trained to measure BP according to a standardized protocol approved by the World Wellness Organization [45]. We used this protocol in our previous randomized trial of storytelling in Vietnam. This protocol was written for use with the OmROn HEM-8712 automatic BP monitor, with special attention to assessment and maintenance of the instrument's accuracy and grooming/certification of research assistants. Using a proper gage size, measurements are taken after sitting quietly for v min, with the arm supported on a apartment surface, with the upper arm at heart level. Three measurements are separated by at least ane min, and values from the last two measurements will be averaged. Summit and weight will be measured in the absence of shoes and heavy clothing while waist and hip sizes will be measured by placing the tape horizontally effectually the smallest role of the waist and the widest portion of the hips, respectively.
Participant surveys
All survey items will be taken from validated instruments, with scales or sub-scales left intact to preserve psychometric backdrop [46]. The survey volition exist implemented in a computer-assisted format and pilot tested for acceptability. The target duration time for the final survey will exist less than 1 h. We will collect information on patient's level of education, occupation, and economic circumstances using the WHO STEPs protocol [47, 48]. STEPs will also be used to collect data on CVD chance factors including tobacco use, alcohol consumption, common salt intake, and concrete activity. Adherence to anti-HTN medications will be measured using standardized forms previously developed past Knuckles University [49]. The Medication Adherence Self-efficacy Scale (MASES) instrument [50, 51] will measure self-efficacy in HTN management. Quality of life will be measured by the short form 12 questionnaire health survey (SF-12) [52]. All the survey measures previously existed in Vietnamese or have been translated and tested by our team as role of our previous work [15] with the exception of the adherence to anti-HTN medications, which will be translated following the gear up of best practices adult past the Us Demography Agency [53]. Translation will be accomplished by a translation team led past study PIs, with multiple versions prepared in parallel followed by team meetings to reconcile possible differences. This approach has been shown to be superior to the simple "back-translation approach" in which translation by a single individual is translated back into the source linguistic communication for review of accuracy [54]. Translation will include cognitive interviews with five participants drawn from the local community. Cerebral testing will identify constructs specific to the Vietnamese linguistic communication and civilization and so that appropriate adjustments may be fabricated to ensure cross-cultural equivalence [55]. Several cycles of revision will exist accomplished at full translation commission meetings conducted in person and by Net video link.
Postal service-DVD viewing interviews for intervention participants will be based on protocols previously adult by our team. The mail-DVD viewing interviews using a structured questionnaire will collect self-reported engagement with the DVDs, including total viewing minutes, specific segments that were viewed, and whether the DVD was shared with family or friends. "Transportation" is a validated concept measuring assimilation into the video story that has been linked to intervention effectiveness and is measured by a validated scale [56, 57]. Participants volition exist asked to elaborate on what motivated/hindered their intervention engagement.
Data direction
Data will be stored on a Wellness Insurance Portability and Accountability Act (HIPAA)-compliant secure server with daily fill-in at the Health Strategy and Policy Institute and managed by the study data manager who is familiar with the REDCap database. This person will work closely with the PIs and experts at University of Massachusetts Medical School to make sure that the data are managed properly.
Report outcomes
Primary outcomes
Change in patient'south systolic BP levels over the one-year follow-up period is the primary trial event. Registered nurses will exist trained and certified to measure patient'due south BP according to a protocol canonical by the World Health Arrangement [45]. Three measurements of BP will be carried out, and values from the last two measurements will be averaged with the commencement reading ignored.
Secondary outcomes
Changes in diastolic BP, risk factors for CVD, medication adherence, self-efficacy, quality of life, toll, and implementation outcomes are secondary outcomes of this study. The WHO STEPs survey, which has been used to investigate the epidemiology of HTN in the Vietnamese language [48], will exist used to collect data on several hazard factors for CVD. Costs include the following: (ane) plan costs, which consist of costs to develop the intervention and implementation costs incurred at the district and community levels, and (2) patient costs such as drugs, diagnostic procedures, time lost, health center visits, and consultation fees.
Implementation outcomes including acceptability, appropriateness, adaptation, feasibility, fidelity, and sustainability will be nerveless via semi-structured interviews and focus grouping discussions amid stakeholders and patient interviews, and post-DVD viewing surveys at follow-upward visits. The implementation data and barriers to study milestones accomplishment gathered by the research team will inform strategies to overcome barriers and progress toward specific study milestones.
Data assay plan
Qualitative analysis
In detail, we will use a well-accepted type of qualitative analyses frequently described as thematic analysis. Thematic assay will be applied to the narratives obtained via Story Development Groups as well every bit from individual interviews from Video Stars and volition go along equally follows: (1) entering transcripts into NVivo (qualitative software), (2) performing initial in vivo coding to separate the full story into initial story units, (3) tagging story units with codes from pre-existing codebook as well equally any additional themes that emerge, and (4) grouping related codes based on broader themes connecting the experiences of participants to key concepts from our conceptual frameworks, which will be explored in the intervention. Thus, our arroyo to thematic assay will include open coding, followed past concept building, and the development of themes/categories [58, 59]. 3 members of the team will code these data—using the Link and Phelan framework [sixty] every bit a guide. Rich data will be gathered from multiple sources to drive the qualitative analyses. Interview notes and recordings from the Story Development Groups and interviews of Video Stars will be linked by a code, and recordings will be destroyed after verification of notes.
For needs assessment report, we will likewise utilize thematic qualitative analysis utilizing the qualitative software platform NVivo to objectively identify and itemize the perceptions expressed in these interviews. The interviews volition be analyzed utilizing a thematic analysis approach to identify key themes every bit they announced in interviews. The identified overarching themes volition serve every bit a baseline to build upon the packaged narratives to be presented visually in the DVDs.
Detailed field notes and codebooks will be maintained. Qualitative data analysis software will support the coding activities for the thematic analysis process higher up and volition allow for inter-rater reliability testing amid multiple coders, who will reach at least a 90% agreement rate.
Quantitative analysis
We will begin the statistical assay by examining univariate statistics, including measures of key tendency and dispersion. We volition carefully document the trial recruitment and retention procedure with a CONSORT diagram [61, 62]. In accord with all-time do, differences in baseline characteristics of the intervention and comparison groups will exist established based on standardized differences, rather than on tests of statistical significance [63, 64].
All chief hypothesis testing will be performed on an intent-to-care for basis and will be two-sided with alpha error will exist set at 0.05. For the chief study hypothesis, the continuous outcomes will exist systolic (H1) and diastolic (H2) BP. We volition employ a generalized linear mixed model adjusting for of import potential covariate imbalances betwixt the ii main study comparison groups. Since we will collect longitudinal information with repeated observations nested within participant, and participants nested inside community, many statistical analyses will be based on a generalized linear mixed model with Restricted Maximum Likelihood (REML) estimation that accounts for the complex information construction through random effects response [65,66,67,68].
We will use mediation analysis to disentangle the multiple mechanisms which may exist associated with the effectiveness of our multi-level intervention. Patient-level mediators of intervention effectiveness include the following: (i) intervention engagement (measured by number of CHW sessions completed and fourth dimension spent in the sessions, types of goals set and respective activeness plans, BP cocky-monitoring, and engagement with the storytelling intervention), (ii) medication adherence, (iii) adherence to heart-healthy lifestyle recommendations, and (4) patient activation. Dispensary-level mediators include implementation of standardized BP measurement protocols, appointment of physicians and nurses in the trial educational programs, and fidelity of CHW intervention delivery.
For the secondary assay, we will examine differential over-time changes in diastolic BP, HTN command, and CVD take a chance, using statistical approaches described higher up for testing the main study hypothesis. For these analyses, a dichotomous measure of BP control will be constructed according to JNC-eight [38]. Overall risk of a CVD issue at ten years volition be calculated based on the Asian Pacific Cohort equation [69]. Nosotros will examine changes in patient's private take a chance factors for CVD (e.chiliad., smoking, physical inactivity) as well. We will acquit out exploratory analyses for possible heterogeneity of intervention result among sub-groups of participants defined by age, sexual practice, and existing CVD.
Missing data may introduce potential bias, and the most of import defense to minimize missing information on primal factors is advanced planning [lxx, 71]. In our previous piece of work, we take developed successful plans to maximize participant retentivity and obtain complete data through sound principles of data collection and quality control [15]. Sensitivity analyses volition estimate the premises of potential bias introduced by omissions. Under the missing-at-random assumption, multiple imputation [72] will generate plausible values of missing covariates while accounting for the additional dubiousness introduced by the omissions. All the analyses will exist performed using STATA xvi.0 (Stata Corp, TX).
The economical analysis volition be led by Dr. Ha, who has expertise in evaluating the cost-effectiveness of interventions to prevent CVD in Vietnam [73]. From the societal perspective, we volition analyze costs and effectiveness for the intervention bundle with the proposed enhancements in comparison to standard implementation of the Vietnam National HTN Program, using approaches appropriate for lower-to-middle income countries [74, 75]. Costs include the following: (i) program costs, which consist of costs to develop the intervention and implementation costs incurred at the commune and community levels, and (two) patient costs such as medications, time lost, health eye visits, and consultation fees. We volition calculate the incremental costs of the intervention past subtracting the boilerplate costs for the intervention group from the average costs for the comparison grouping. The incremental price-effectiveness ratio will be calculated equally the boosted price of the intervention divided by the alter in both systolic and diastolic BP related to the intervention. In addition, we volition calculate cost-effectiveness ratios for BP control, past dividing the additional costs of the intervention past the proportion who achieved HTN control as a consequence of the intervention. Because this is a country-specific economic analysis, all costs will be evaluated using the Vietnamese Dong (VND) with subsequent conversion to US dollars. We will perform one-way sensitivity analyses related to variable intervention effectiveness, variable engagement, and trial adherence.
Timeline
The proposed study will take place over a 5-twelvemonth menses. During the first 6 months of year 1, we volition conduct a needs assessment study. The next 18 months will be devoted for intervention enhancement development. Grooming for intervention delivery will be conducted in early yr 3, right before the trial showtime (twelvemonth 3). All data drove activities for the trial will be completed by mid-year 5. Yr five will be devoted to data analysis and manuscript writing.
Trial oversight
The trial steering commission is equanimous of all PIs and study fundamental personnel from all sites. This committee is responsible for designing and implementing the study and recommends appropriate actions to ensure that protocol deviations will exist minimized. An independent Information Condom Monitoring Lath (DSMB) has been established to be responsible for safeguarding the interests of study participants, assessing the condom and efficacy of report procedures, and monitoring the overall conduct of the study. Communication with DSMB members will exist primarily through the DSMB ambassador (Executive Secretarial assistant—ES) and the Data Coordinating Center (DCC). The first DSMB meeting volition be held before the trial showtime to review written report protocol and give approving and will meet every 6 months to reviewing data for rubber and feasibility. Additional meetings will be arranged every bit needed. An interim analysis will not be performed. The DSMB may brand recommendations whether the study/the trial will be connected or stopped. The DSMB will also receive all Serious Agin Event (SAE) reports and may request additional data, every bit needed. In improver, on-site monitoring visits from a qualified research monitor will exist scheduled quarterly until data quality is accounted acceptable and and so will be scheduled 6 monthly for the residuum of the study. The report protocol conforms to the SPIRIT checklist (Boosted file 1).
Broadcasting plan
The project will work closely with policymakers from the Vietnam Ministry building of Wellness to transform bear witness into a policymaking procedure also as with the Vietnam Eye Association to scale upwardly the interventions and implementation program and make them more widely available. The process will start with a dissemination workshop and and so forums for policy dialogue with participation of key leaders and influential people from the Ministry of Health, policymakers, health managers, practitioners, and stakeholders who are involved with the prevention and control of non-catching diseases in Vietnam. The results of the trial will be published in international peer-reviewed journals by the study squad.
Word
We are proposing a total-scale cluster RCT to test the effectiveness of two strategies to better HTN control for adults residing in rural communities in Vietnam. We volition use a well-planned approach to culturally adapt intervention enhancements that take been effective in our feasibility trial and from other settings. If our arroyo is proven to be successful, it will offering policymakers an innovative intervention strategy to address a well-recognized and emerging threat to public health in Vietnam. Our arroyo is built on many previous "lessons learned" and, more than importantly, is low cost and of low burden to patients, clinicians, and health care systems.
However, there are some potential study caveats. Due to the nature of the study design, the trial is not blinded. With 12 months of follow-upwardly, nosotros are unable to appraise the long-term furnishings of the intervention on CVD morbidity and mortality. Too, given limitations of sample size, we are not testing each intervention component separately. Still, mediation analysis and assay of implementation data will assistance uncrease the contributions of specific intervention components. Another potential threat is contamination of the comparison group, if the storytelling DVDs or abode BP monitors are shared. Thus, we specified that the distance between an intervention and comparison customs volition be at least 10 km. Evidence of contagion will be sought through patient interviews at the last study visit.
Patient recruitment to the trial may lag, or the intervention may non be faithfully implemented. We recognize the demand for proactive monitoring and response, and volition implement previously developed protocols that were successful in rural Vietnam. Implementation data with specific milestones volition provide early warnings to guide corrective action. We volition work with the NIH Scientific Officer and the structure for coordination across studies to be funded under the Hypertension Outcomes for T4 Research inside Lower Center-Income Countries (Hy-TREC) initiative for scientific guidance as appropriate and to establish meaningful study milestones.
The "Conquering Hypertension in Vietnam: Solutions at Grassroots Level" will provide new evidence about the effectiveness and implementation of the Vietnam National HTN Plan, which is being rolled out beyond the nation merely has not been role of a randomized trial in its electric current format, and will evaluate our proposed enhancements to this ambitious national attempt.
Trial status
The recruitment began on October 23, 2019, and will complete on March 1, 2021.
To date, randomization has been finished and 423 patients have been recruited from 10 communes (updated on October eighteen, 2020). Xc-7 percent of patients completed the three-calendar month follow-up contact (211/218 eligible patients), and nearly 90% take completed the six-month follow-up contact (84/94 eligible patients). This protocol is version 6, dated August 15, 2019. Any protocol modifications will be communicated to relevant parties (e.g., IRB, trial participants) and published on relevant channels (e.grand., ClinicalTrials.gov).
Availability of data and materials
The datasets, informed consent class, and other study materials of the current study will exist available from the corresponding author on reasonable request. All data will exist de-identified before sharing.
Abbreviations
- BP:
-
Blood pressure
- CHC:
-
Community health eye
- Consort:
-
Consolidated Standards of Reporting Trials
- CVD:
-
Cardiovascular illness
- DVD:
-
Digital video disc
- HTN:
-
Hypertension
- ICC:
-
Intra-form correlation coefficient
- JNC:
-
Articulation National Committee
- RCT:
-
Randomized controlled trial
- WHO:
-
World Health Organization
References
-
Vietnam Ministry of Health. Health Statistics Yearbook 2018, Ministry of Health, Hanoi, Vietnam. 2018.
-
Nguyen TT, Hoang MV. Non-communicable diseases, food and nutrition in Vietnam from 1975 to 2015: the burden and national response. Asia Pac J Clin Nutr. 2018;27(i):19–28.
-
Jamison DT, Breman JG, Measham AR, et al. Illness control priorities in developing countries. Second edition. Washington DC: World Banking company Publications; 2006. p. 20433.
-
Hoang VM, Dao LH, Wall S, et al. Cardiovascular disease mortality and its clan with socioeconomic status: findings from a population-based cohort study in rural Vietnam, 1999-2003. Prev Chronic Dis. 2006;3(3):A89.
-
Ministry of Health. Joint almanac wellness review 2014-strengthening prevention and control of non-catching affliction. Hanoi: Medical Publishing Business firm; 2015.
-
Son PT, Quang NN, Viet NL, et al. Prevalence, awareness, handling and control of hypertension in Vietnam-results from a national survey. J Hum Hypertens. 2012;26(4):268–lxxx.
-
Tran QB, VM, Hoang HL Vu, et al., Gamble factors for Not-Communicable Diseases among adults in Vietnam: Findings from the Vietnam STEPS Survey 2015. J Glob Wellness Sci. 2020;two(1).
-
Ha DA, Goldberg RJ, Allison JJ, et al. Prevalence, awareness, handling, and control of high blood pressure level: a population-based survey in Thai Nguyen, Vietnam. PLoS One. 2013;8(6):e66792.
-
Dower C, Knox M, Lindler V, et al. Advancing customs health worker practice and utilization: the focus on financing. San Franciso, CA: National Fund for Medical Education/The Eye for Health Professions; 2006.
-
Alexander JA, Hearld LR. Methods and metrics challenges of delivery-system research. Implement Sci. 2012;7:15.
-
Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of customs health workers in the care of people with hypertension. Am J Prev Med. 2007;32(5):435–47.
-
Turner BJ, Hollenbeak CS, Liang Y, et al. A randomized trial of peer omnibus and office staff support to reduce coronary heart affliction run a risk in African-Americans with uncontrolled hypertension. J Gen Intern Med. 2012;27(x):1258–64.
-
Hurtado Yard, Spinner JR, Yang G, et al. Knowledge and behavioral effects in cardiovascular health: community health worker health disparities initiative, 2007-2010. Prev Chronic Dis. 2014;11:E22.
-
Nguyen HL, Ha DA, Goldberg RJ, et al. Culturally adaptive storytelling intervention versus didactic intervention to improve hypertension control in Vietnam- 12 calendar month follow up results: a cluster randomized controlled feasibility trial. PLoS One. 2018;13(12):e0209912.
-
Nguyen HL, Allison JJ, Ha DA, et al. Culturally adaptive storytelling intervention versus didactic intervention to improve hypertension control in Vietnam: a cluster-randomized controlled feasibility trial. Pilot Feasibility Stud. 2017;3:22.
-
Allison JJ, Nguyen HL, Ha DA, et al. Culturally adaptive storytelling method to improve hypertension command in Vietnam - "We talk about our hypertension": study protocol for a feasibility cluster-randomized controlled trial. Trials. 2016;17(1):26.
-
Agyemang C, Bruijnzeels MA, Owusu-Dabo E. Factors associated with hypertension sensation, treatment, and control in Ghana, W Africa. J Hum Hypertens. 2005;twenty(1):67–71.
-
Muntner P, Gu D, Wu X, et al. Factors associated with hypertension awareness, treatment, and control in a representative sample of the Chinese population. Hypertension. 2004;43(3):578.
-
Cooper LA, Roter DL, Bone LR, et al. A randomized controlled trial of interventions to heighten patient-md partnership, patient adherence and loftier blood pressure control amidst ethnic minorities and poor persons: study protocol NCT 00123045. Implement Sci. 2009;4(1):seven.
-
Stergiou GS, Ntineri A, Kollias A. Changing human relationship amidst office, ambulatory, and home claret pressure with increasing age: a neglected issue. Hypertension. 2014;64(5):931–2.
-
Margolis KL, Asche SE, Bergdall AR, et al. Issue of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46–56.
-
McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high run a risk of cardiovascular affliction: the TASMIN-SR randomized clinical trial. JAMA. 2014;312(8):799–808.
-
Uhlig K, Patel Grand, Ip S, et al. Cocky-measured blood force per unit area monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185–94.
-
Ralston JD, Cook AJ, Anderson ML, et al. Home blood pressure monitoring, secure electronic messaging and medication intensification for improving hypertension command: a mediation analysis. Appl Clin Inform. 2014;5(1):232–48.
-
van Onzenoort HA, Verberk WJ, Kroon AA, et al. Issue of cocky-measurement of blood pressure level on adherence to treatment in patients with mild-to-moderate hypertension. J Hypertens. 2010;28(three):622–seven.
-
Weiner BJ. A theory of organizational readiness for modify. Implement Sci. 2009;iv:67.
-
Adair R, Christianson J, Wholey DR, et al. Care guides: employing nonclinical laypersons to assistance main care teams manage chronic disease. J Ambul Care Manage. 2012;35(1):27–37.
-
Wagner EH. Chronic disease management: what will it take to ameliorate treat chronic disease? Eff Clin Pract. 1998;1(1):2–four.
-
Coleman K, Austin BT, Brach C, et al. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood). 2009;28(1):75–85.
-
Cheng EM, Cunningham WE, Towfighi A, et al. Randomized, controlled trial of an intervention to enable stroke survivors throughout the Los Angeles County condom net to "stay with the guidelines". Circ Cardiovasc Qual Outcomes. 2011;4(2):229–34.
-
Dolor RJ, Yancy WS Jr, Owen WF, et al. Hypertension Comeback Project (HIP): study protocol and implementation challenges. Trials. 2009;ten:xiii.
-
Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53.
-
Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into do. Jt Comm J Qual Patient Saf. 2008;34(4):228–43.
-
Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services enquiry findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
-
Kitson AL, Rycroft-Malone J, Harvey Thou, et al. Evaluating the successful implementation of testify into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci. 2008;3:i.
-
Rycroft-Malone J. The PARIHS framework--a framework for guiding the implementation of evidence-based practise. J Nurs Care Qual. 2004;19(four):297–304.
-
Stetler CB, Damschroder LJ, Helfrich CD, et al. A guide for applying a revised version of the PARIHS framework for implementation. Implement Sci. 2011;six:99.
-
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American Higher of Cardiology/American Heart Association Task Strength on Clinical Exercise Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248.
-
Vietnam Ministry of Health. National Strategy on Prevention and Control of Cancer, Cardiovascular Disease, Diabetes, Chronic Obstructive Pulmonary Disease, Asthma, and Other Non-Commnicable Diseases: Period 2015-2025. Hanoi: Ministry of Health; 2016.
-
Miller WR, Rollnick S. Motivational interviewing: preparing people for change (2nd ed.). New York: The Guilford Press; 2002.
-
Greaves CJ, Middlebrooke A, O'Loughlin L, et al. Motivational interviewing for modifying diabetes hazard: a randomised controlled trial. Br J Gen Pract. 2008;58(553):535–40.
-
Naar-King S, Outlaw A, Dark-green-Jones Yard, et al. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–73.
-
Donner A, Klar N. Statistical considerations in the blueprint and analysis of community intervention trials. J Clin Epidemiol. 1996;49(4):435–9.
-
Fritz MS, Mackinnon DP. Required sample size to find the mediated result. Psychol Sci. 2007;18(3):233–9.
-
WHO, STEPS survey. https://www.who.int/ncds/surveillance/steps/STEPS_Manual.pdf. Accessed 17 Mar 2020.
-
Rust J, Golombok Due south. Modern psychometrics, third edition: the scientific discipline of psychological cess. New York, NY: Routledge; 2009.
-
Bonita R, De Counter M, Dwyer T, et al. Surveillance of risk factors for noncommunicable disease: the WHO STEPwise arroyo. Geneva: World Health Organization; 2001.
-
Minh HV, Byass P, Chuc NT, et al. Gender differences in prevalence and socioeconomic determinants of hypertension: findings from the WHO STEPs survey in a rural community of Vietnam. J Hum Hypertens. 2006;20(2):109–15.
-
Voils CI, Maciejewski ML, Hoyle RH, et al. Initial validation of a self-written report measure out of the extent of and reasons for medication nonadherence. Med Care. 2012;50(12):1013–9.
-
Fernandez Southward, Chaplin W, Schoenthaler AM, et al. Revision and validation of the medication adherence self-efficacy scale (MASES) in hypertensive African Americans. J Behav Med. 2008;31(6):453–62.
-
Ogedegbe G, Mancuso CA, Allegrante JP, et al. Evolution and evaluation of a medication adherence self-efficacy scale in hypertensive African-American patients. J Clin Epidemiol. 2003;56(6):520–9.
-
Ware J Jr, Kosinski Thou, Keller SD. A 12-Item Short-Form Health Survey: structure of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33.
-
Pan Y, de la Puente One thousand. Census Agency guideline for the translation of data drove instruments and supporting materials: documentation on how the guideline was developed; 2005.
-
Behling O, Police KS. Translating questionnaires and other research instruments: problems and solutions. London: Sage publications, Inc; 2000.
-
Census Bureau Standard: Pretesting Questionnaires and Related Materials for Surveys and Censuses. 2003. https://world wide web.census.gov/srd/pretest-standards.html.
-
Greenish MC. Transportation into narrative worlds: the office of prior noesis and perceived realism. Discourse Process. 2004;38(2):247–66.
-
Green MC, Brock TC. The part of transportation in the persuasiveness of public narratives. J Pers Soc Psychol. 2000;79(5):701–21.
-
Strauss A, Corbin J. Basics of qualitative enquiry: grounded theory, procedures, and techniques. Newbury Park, CA: Sage Publications; 1990.
-
Denzin NK, Lincoln YS. The discipline and do of qualitative enquiry, in Handbook of Qualitative Research: Sage publications, Thousand Oaks, CA; 2000.
-
Link BG, Phelan JC. Conceptualizing stigma. Annu Rev Sociol. 2001;27(1):363–85.
-
Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–94.
-
Moher D, Hopewell Due south, Schulz KF, et al. Espoused 2010 caption and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
-
Austin PC, Manca A, Zwarenstein Yard, et al. A substantial and disruptive variation exists in treatment of baseline covariates in randomized controlled trials: a review of trials published in leading medical journals. J Clin Epidemiol. 2010;63(2):142–53.
-
Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;xiii(17):1715–26.
-
Albert PS. Longitudinal data analysis (repeated measures) in clinical trials. Stat Med. 1999;18(thirteen):1707–32.
-
Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford: Clarendon Press; 1995.
-
Edwards LJ. Mod statistical techniques for the assay of longitudinal data in biomedical research. Pediatr Pulmonol. 2000;xxx(iv):330–44.
-
Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. Higher Station, TX: State Press; 2005.
-
Asia Pacific Cohort Studies, C, Barzi F, Patel A, et al. Cardiovascular take chances prediction tools for populations in Asia. J Epidemiol Community Wellness. 2007;61(2):115–21.
-
Groenwold RH, Donders AR, Roes KC, et al. Dealing with missing effect data in randomized trials and observational studies. Am J Epidemiol. 2012;175(iii):210–7.
-
Fiddling R, Rubin D. Statistical analysis with missing data, second edition. New York: John Wiley and Sons; 2002.
-
Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):three–15.
-
Ha DA, Chisholm D. Cost-effectiveness analysis of interventions to prevent cardiovascular disease in Vietnam. Health Policy Programme. 2011;26(3):210–22.
-
Hutton One thousand, Baltussen R. Cost valuation in resources-poor settings. Health Policy Programme. 2005;20(iv):252–9.
-
Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;i(1):eight.
Acknowledgements
Non applicable.
Funding
Research reported in this article was supported past the US National Heart, Lung, and Blood Institute of the National Institutes of Health under honour number 1U01HL138631-01. The views expressed are those of the authors and practise not necessarily correspond those of the National Eye, Lung, and Blood Institute, the National Institutes of Health, the Department of Wellness and Homo Services, or the Us Regime. This funding agency had no role in the design of the study and drove, analysis, and estimation of information and in writing the manuscript.
Writer data
Affiliations
Contributions
JJA, RJG, HLN, DAH, and OTT conceived the study and participated in its pattern. GC, VHP, CTN, GHN, HVP, TTN, and TTL participated in the development of the report protocol. All authors read and approved the final manuscript.
Respective author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board at the Health Strategy and Policy Found (HSPI) in Hanoi, Vietnam (Decision 171/QD-CLCSYT), canonical this written report. Written informed consent to participate volition be obtained from all participants.
Consent for publication
NA
Competing interests
The authors declare that they accept no competing interests.
Additional data
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents.
Rights and permissions
Open Admission This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, accommodation, distribution and reproduction in any medium or format, as long equally you give appropriate credit to the original author(south) and the source, provide a link to the Creative Commons licence, and indicate if changes were fabricated. The images or other third party cloth in this article are included in the article'south Creative Commons licence, unless indicated otherwise in a credit line to the fabric. If material is not included in the article'southward Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data fabricated available in this article, unless otherwise stated in a credit line to the data.
Reprints and Permissions
About this commodity
Cite this commodity
Ha, D.A., Tran, O.T., Nguyen, H.Fifty. et al. Conquering hypertension in Vietnam—solutions at grassroots level: study protocol of a cluster randomized controlled trial. Trials 21, 985 (2020). https://doi.org/ten.1186/s13063-020-04917-8
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s13063-020-04917-eight
Keywords
- Hypertension
- Cocky-monitoring blood force per unit area
- Storytelling
- Trial
- Vietnam
Hypertension Treatment Simulation Software Download Pc Software
DOWNLOAD HERE
Source: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-020-04917-8
Posted by: weibelanwave.blogspot.com